| NO. | Name | Specification and Composition | Consumption quota | Remark |
| 1 | Alcohol | ≥95% | 1.170t/t | |
| 2 | Demineralized Water | Pure Deionized Water | 4.3t/t | |
| 3 | Process Water |
25PPM(5℃) Chloridion |
6t/t | |
| 4 | Vapour | 0.8MPa | 3.0t/t | |
| 5 | Instrument Compressed Gas | 0.6MPa | 7m3/t | |
| 6 | Circulating Cooling Water | Water Inlet30℃ Water Outlet38℃ Water Pressure 0.35MPa | 410t/t | |
| 7 | Chilled Water | Water Inlet7℃, Water Outlet12℃ | 160×104KJ/t | |
| 8 | Electric | 380V 50Hz | 250KWH/t | |
| 9 | Waste Water | Ethanol 0.03% Acidity 0.25% COD5000ppm BOD2000ppm | 5t/t | |
| 10 | Exhaust Gas | Acetaldehyde≤0.1% CO+CO2 ≤0.8% H2+ CH4 ≤1.2% H2O≤1% N2≥96% | 1.143t/t | |
| 11 | Acetaldehyde Yield | 99.7%wt |
| Project | Type and parameter | Production line | Finish Date |
|---|---|---|---|
| Shandong Hongda Biotechnology Co., LTD | Full set of 20k tpa Acetaldehyde non-standard equipment and technical service | 1 | 2009.3 |
| Shandong kunda Biotechnology Co., LTD | Full set of 20k tpa Acetaldehyde non-standard equipment and technical service | 1 | 2010.3 |
| Ordos Hongde Chemical Co.,ITD |
80kt /a formaldehyde customized equipment and technical service 60k tpa Acetaldehyde customized equipment and technical service |
2 | 2010.7 |
| Tangshan Chenhong Industrial Co., LTD | 40k tpa Acetaldehyde customized equipment and technical service | 1 | 2013.04 |
| Anhui Guoxing Biochemistry Co.,LTD | Whole set of 60k tpa Acetaldehyde non-standard equipment and technical service | 2 | 2014.06 |
Acetaldehyde is a basic organic material for chemical industry. It is mainly used for production of acetic acid, ethyl acetate and acetic anhydride, and it is also used to make Pentaerythritol, crotonaldehyde, crotonic acid and chloral hydrate.
There are three methods for making acetaldehyde, first is acetylene hydration process, and second is ethylene oxidation process, and third is alcohol oxidation process. At present, alcohol oxidation process is adopted by most of the acetaldehyde production plants of our company, to make acetaldehyde. The main reason is that the alcohol material is with lower price and extensive resources. But the alcohol oxidation process has low conversion rate of alcohol. The acetaldehyde production plants of our company are very advanced, by adopting double towers for absorption, with one tower for circulation absorption, double tower for spraying absorption and recycling plant, for making the best consumption.
Alcohol oxidation process is that alcohol makes acetaldehyde and hydrogen with silver catalyst, and hydrogen has reaction with the oxygen in the air, to make water and push the reaction to the right. But oxidation is a complex reaction, which can not only oxidize hydrogen to water, but also make alcohol and acetaldehyde have further oxidation, turning into CO2 and H2O.In the real production, oxygen is limited to supply. Usually the ratio of oxygen and alcohol is controlled to be about 0.30-0.35, to keep the hydrogen caused by dehydrogenation unable to be oxidized to water in time, and hydrogen can still exist in reaction gas. The whole process can be seen as dehydrogenation oxidation. 80% hydrogen caused by dehydrogenation has been oxidized to water. It is traditionally said that 80% acetaldehyde is made from the oxidation reaction. 20% acetaldehyde is made from the dehydrogenation. The dehydrogenation is an endothermic process. And the oxidation reaction is exothermic process. For oxidation rate is greater than dehydrogenation rate, the heat release is greater than heat absorption, so the whole process is the exothermic process. For alcohol process to make acetaldehyde, except the oxidation process, the copper catalyst process for dehydrogenation can also be used. The dehydrogenation process is the endothermic process. Reactor needs heating. The oxidation process is the exothermic process, and heat can be recycled to produce steam. The plant costs and operating costs of dehydrogenation process are higher than oxidation process. Thus except for special requirements for the hydrogen and higher usage, oxidation process is better than the dehydrogenation.
Acetylene hydration process is operated under condition of 98~100℃ and 150000Pa, acetylene gas made is put to sulfuric solution with mercuric sulfate. The acetylene and water has addition reaction under mercuric sulfate catalyst, making ethenol. For ethenol is very unstable, it will have rearrangement in time to become acetaldehyde, and this reaction is called Coetzee's reaction. This reaction has very complicated process. It is thought by someone that maybe the alkynes PI and mercury ions generates PI - complex, then PI – complex has hydrolysis, producing ethylene glycol, and then rearrangement of acetaldehyde.
1.The process basic principle
Reaction principle
Alcohol makes acetaldehyde and hydrogen with silver as catalyst. Hydrogen and oxygen in air has oxidation, making water, and push the reaction to the right. But oxidation reaction is a complex reaction, which can not only oxidize hydrogen to water, but also makes alcohol and acetaldehyde further oxidize to CO2 and H2O. In the real production, oxygen is limited to supply. Usually the ratio of oxygen and alcohol is controlled between 0.30 and 0.35, to stop hydrogen caused by dehydrogenation from turning into water. There is still hydrogen in the reaction air. The whole process is the dehydrogenation oxidation reaction. 80% of hydrogen produced from dehydrogenation is oxidized into water. It is traditionally said 80% acetaldehyde is produced from oxidation, and 20% acetaldehyde is produced from dehydrogenation. The dehydrogenation is endothermic process and oxidation is exothermic process. Because the oxidation rate is bigger than dehydrogenation rate and the exothermic value is bigger than endothermic value. So the whole process is exothermic process.
Main reaction:
CH3CH2OH+1/2O2=CH2CHO+H2O+173.1kJ/mol
CH3CH2OH=CH3CHO+H2-68.9 kJ/mol
H2+1/2O2=H2O+242kJ/mol
In addition, owing to the reaction condition changing, the following one or several side reactions can happen:
CH3CH2OH+O2→CH3COOH+H2O
CH3CH2OH+3 O2→2CO2+3H2O
CH3CHO→CH4+CO
CO+1/2 O2→CO2
2CH3CH2OH →CH3COOC2H5
CH3CH2OH+1/2 O2→2C+2H2O+H2
To produce acetaldehyde with alcohol, except the oxidation, the dehydrogenation with copper as catalyst can be adopted. The dehydrogenation process is endothermic process, with reactor needing heating. The oxidation process is the exothermic process; the heat can be recycled to produce steam. The costs for equipments and operation of dehydrogenation are higher than those of oxidation process.
Absorption theory
Acetaldehyde absorption system adopts the double tower absorption. The absorption quantity of the second tower (that is the water adding quantity of the second tower) is the main controlling method to control acetaldehyde concentration. In operation, the higher spraying concentration is usually controlled, to keep the packing in the tower having complete wet surface. The gas phase and liquid phase will have complete contact to enhance the absorption affection.
The other purpose of controlling the tower liquid quantity is to control the liquid level of the tower bottom, to keep it in a certain range. The too high liquid level of the tower bottom may exceed the gas phase pipe entry, which will make the reacted gas unable to come into tower, highly increase the system resistance, or have accident of roots blow being switched off. The too low or empty tower liquid level will cause no output of circulation liquid of the circulation pump, and the back flowing of the tower will be slowly decreased until the spraying comes out, which will cause the high temperature of the tower and the interrupted absorption, and the acetaldehyde gas discharged will have environmental pollution. The operation requires the balance between liquid input and output to control the flow meter of controlling tower
The rectification theory。
It is called rectification by using the volatile differences of components with backflow of liquid phase and gas phase, to make gas phase and liquid gas countercurrent multistage, then to move the difficultly volatile components from gas phase to liquid phase, and make mixture continuously separating.
In this process, heat transfer and mass transfer process are carried out simultaneously which is controlled by mass transfer process. Raw materials come into the reaction tower from the suitable section of tower’s middle part to divide the tower into two parts. Upper is rectifying section, excluding feed. The down part is stripping section containing the feed plate. The condenser provides a liquid reflux from the top of the tower, the reboiler provide gas reflux from the bottom of the tower. Gas and liquid reflux is the important characteristic of rectification
2. Device technical characteristics
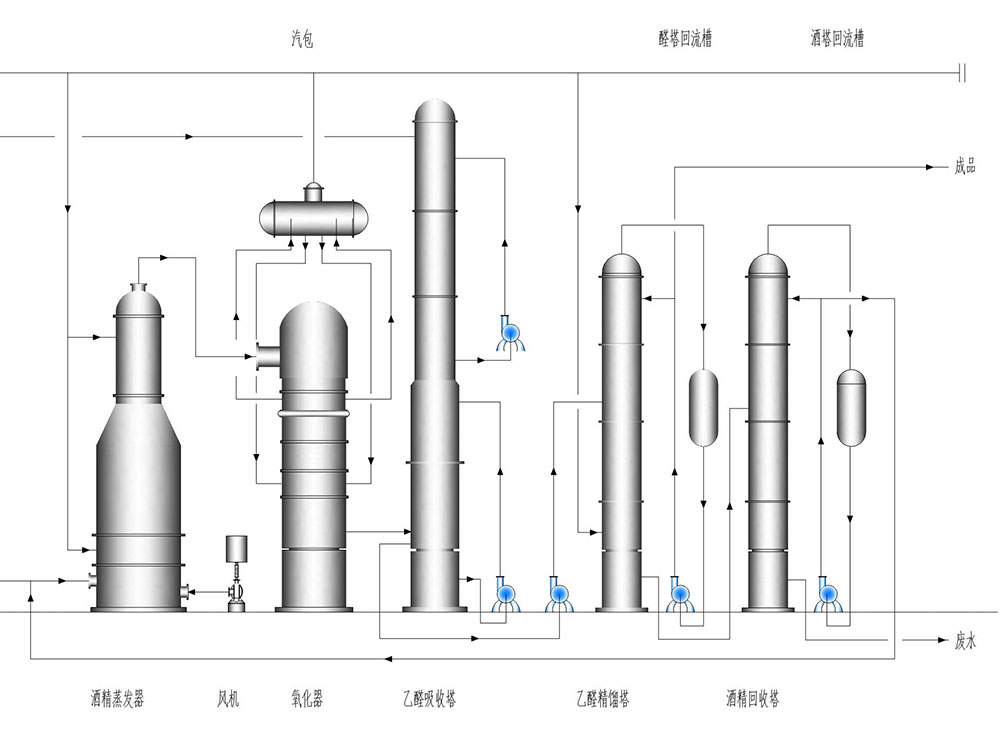
This plant adopts that raw material alcohol and the recycled alcohol, after mixing in mixer, comes into evaporator, then forms mixture with air, and comes into reactor for reaction. The reaction heat forms steam, preheating boiler feeding water and dilute acetaldehyde feeding. Four-section of Twin Towers is adopted in absorption. Acetaldehyde distillation uses pressurized operation. The wastewater generated by the liquor tower is used to heat the alcohol in the evaporator. Therefore, the energy saving effect of the process is obvious, and the investment of the plant is reduced.
Process description
Alcohol mixtureBefore feeding, an accurate analysis of the concentration of material alcohol and recycled alcohol is carried out by analyst. According to the preparation concentration, it is calculated the required volume ratio of raw material alcohol and alcohol recovery, or soft water. The calculated numerical is inputted into computer. The frequency converter of feeding pump is used to control ratio of material alcohol and the recycled alcohol. The liquid level of evaporator is adjusted by the feeding pump as well.
Air transportation
After removing impurities like dust by air filter, the outside air comes into evaporator via roots blower for increasing pressure and air distributor.
Steam oxidation
The alcohol made in evaporator comes into superheater under effect of roots blower and steam heating. It is heated by outside steam to 120 degrees Celsius above, and it comes into reactor via fire arrester filter. It has rapid reaction with electrolytic silver catalyst. Temperature of the reactor is stabilized by controlling the evaporation temperature. The mixture after reaction comes into acetaldehyde preheater together with saturation hot water and steam drum inlet water in turn.
Cooling absorption
The reaction gas coming from reactor is cooled to be below 20℃ via diluted acetaldehyde preheater, the first grade furnace gas water cooler and the two - stage furnace gas salt cooler in turn, then it comes into the first absorber. The first absorber adopts two-stage circulation cooling absorption and the gas which was not absorbed into the second absorption tower. The second absorption tower bottom is cycle absorption and soft water is added to its top part for natural absorption. The exhaust gas from the top gets directly emptying. The absorbing liquid of second absorber returns to the upper part of the first absorber. The absorbing liquid of the upper part of the first absorber is returned to the lower part of the first absorber. The concentration of the absorbing liquid in the lower part is controlled between 10-18%. Control the appropriate liquid level of the absorbing liquid and the extra absorption liquid is put into dilute acetaldehyde tank.
The dilute acetaldehyde comes into the dilute acetaldehyde preheater and aldehyde tower feeding heater and the top of the acetaldehyde tower(The feeding port of the aldehyde tower is divided into middle and top part) in turn. At the same time,reboiler of the acetaldehyde tower is heated by the steam from outside, to control the temperature and reflux ratio of acetaldehyde in the top tower to obtain the high purity acetaldehyde. Acetaldehyde tower top gas is condensed into liquid by circulating water secondary cooling. The non condensable gas gets continuously condensed via the aldehyde column condenser. The off gas is sent to absorption tower, and qualified concentrated acetaldehyde is sent to concentrated acetaldehyde storage tank.
Alcohol recovery
Acetaldehyde tower bottom liquid is pressed into alcohol recycling tower under effect of acetaldehyde tower internal pressure, and the tower bottom is heated by steam. Alcohol recovery tower temperature and reflux ratio is controlled to obtain certain concentration of alcohol recovery. The gas of the top tower is condensed by circulating water. The condensed liquid gets partial circulation under effect of pump. Parts comes into recycled alcohol cooler, getting heat exchanging with circulation water, and comes into recycled alcohol tank. The distillation residual liquid is discharged under effect of alcohol tower residual liquid pump.






 Layout Drawing
Layout Drawing