Formaldehyde (HCHO) is a basic raw material in chemical industry, which is widely used in the production of resin, plastic, leather, paper and fiber. It plays an extremely important role in people's daily life. China's formaldehyde industry started in 1956. After more than 60 years of continuous improvement, research and further development, China has become the world's largest producer and consumer of formaldehyde.
On the basis of traditional formaldehyde production technology, Jiangsu Dolton petrochemical technology co., Ltd., with a large number of senior chemical engineers and their continuous innovation, has developed off gas cycling technique, no alcohol low transformation process, flue gas circulation process and the latest silver catalyzed formaldehyde technology. It has made its presence felt in the development of China formaldehyde technology, acting as the leader of china formaldehyde industry.
Metal Oxide Formaldehyde
Metal oxide formaldehyde is operated under the explosive lower limit of methanol-air, that is, under the condition of excessive air, so it is also called “air excess method”. This reaction is carried out under normal pressure and 250℃-400℃, which can reduce the side reaction, increase the conversion rate and reduce the methanol consumption.
Main Reaction CH3OH+1/2O2→CH2O+H2O
Side Reaction CH3OH+3/2O2→CO2+2H2O
CH3OH+O2→CO+2H2O
CH3OH+O2→HCOOH+H2O
2CH3OH→CH3OCH3+H2O
Process Description
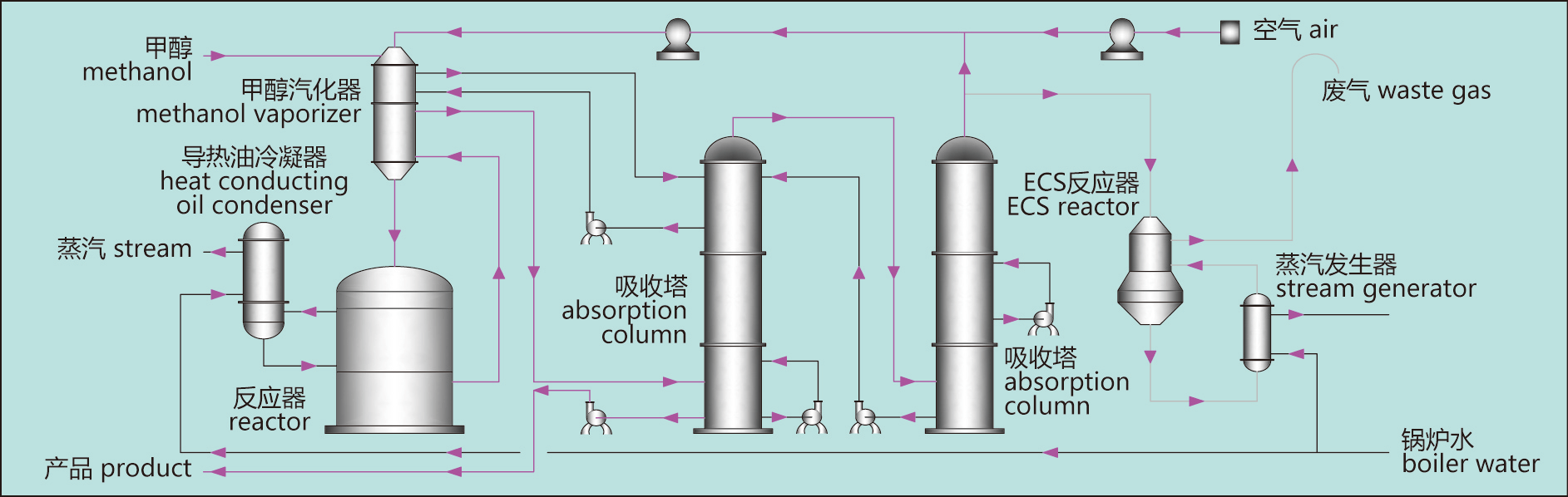
Metal oxide formaldehyde production consists of gas compression, methanol vaporization, methanol oxidation, formaldehyde absorption and off gas treatment.
Gas compression: after the air is filtered and pressurized by the fresh air blower, it is evenly mixed with the circulating off gas returned from the absorption tower to form the mixture gas. The mixture is pressurized to a suitable pressure by a circulating blower and sent to the top of the methanol vaporizer.
Methanol vaporization: methanol from the raw material tank enters the methanol vaporizer, which is atomized into the mixture through the atomizing nozzle, and vaporizes and overheats through the methanol vaporizer from top to bottom. The heat sources of the upper and lower parts of the methanol vaporator are circulating solution in column one and the high-temperature gas at the outlet of the reactor.
Methanol oxidation: the superheated raw gas enters the top of the reactor. The reactor has a heat exchange tube containing iron and molybdenum catalyst. Methanol reacts with oxygen under the action of catalyst to produce formaldehyde and give off a lot of heat. The heat of reaction vaporizes the heat conducting oil within the reactor. After vaporization, the heat conduction oil is condensed in the condenser and returned to the reactor while the by-product steam is produced.
Formaldehyde absorption: the high-temperature mixture from the bottom of the reactor first passes through the methanol vaporator for cooling, and then goes into the absorption section for recycling absorption. The absorption section is divided into two formaldehyde absorption columns. Qualified formaldehyde products are extracted from the bottom of the absorption column one.
Off gas treatment: absorb the off gas from the top of the second column, and some of it will return to the fresh air blower outlet, and the other part will enter the ECS reactor. In off gas, carbon monoxide, dimethyl ether and methanol react with precious metal catalyst to produce carbon dioxide and water, and the by-product steam is sent out.
Metal Oxide process flow chart
Consumption
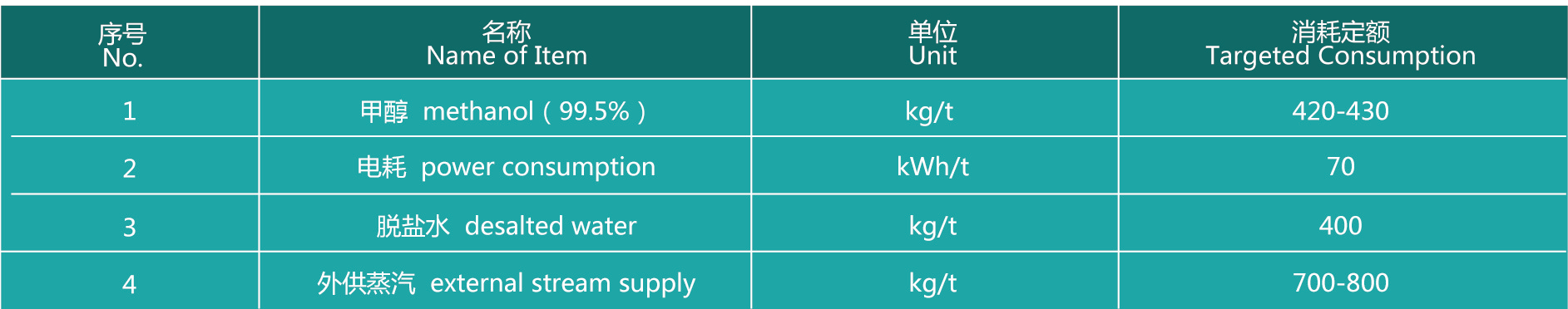
Metal oxide process consumption index
Process principle and characteristics
Using refined methanol as raw material and electrolytic silver as catalyst, oxidation dehydrogenation reaction takes place at 630℃~670℃ to produce formaldehyde, and then pure water (or desalted water) is absorbed in the absorption column to produce 37.0%~55.0% formaldehyde solution. At methanol excess and specified reaction temperature, there are following main reactions:
CH3OH + 1/2O2 → HCHO + H2O - 156.557KJ/mol ①
CH3OH ↔ HCHO + H2 +85.270KJ/mol ②
The reaction ① starts at 200℃, and the reaction ② is reversible at about 600℃. The generated hydrogen and oxygen react to generate water, so that the reaction ② continues to move rightward, waste heat in the reaction is taken away by batching steam and off gas, so that the reaction temperature is kept constant. This reaction is operated above the upper limit of methanol explosion, and the following side reactions occur at the same time.
CH3OH + 3/2O2 → CO2 + 2H2O -675.99KJ/mol ③
HCHO + O2 → CO2 + H2O -393.01KJ/mol ④
HCHO + l/2O2 → HCOOH -246.73KJ/mol ⑤
|→ CO + H2O
CH3OH + H2 → CH4 + H2O -115.51KJ/mol ⑥
In the above reaction ② is the endothermic reaction, only at high temperature the reaction rate was significantly improved. And ①, ③, ④, ⑤, ⑥ are exothermic reaction, the whole reaction system is in the state of excess heat energy.
Latest silver catalyzed formaldehyde process
Off gas cycling process and low conversion alcohol free process are developed on the basis of traditional technology. The processing route is: methanol evaporation - overheating – filtering - oxidation - 1 # absorption column - 2 # absorption column (3 # absorption column) - off gas burning. This process has a long processing route, more equipments, high energy consumption and big occupation of land. On the basis of many years practice keeping the traditional processing method, Dolton has developed its own proprietary technology, the latest process of silver catalyzed formaldehyde production.
Technical flow and its characteristics
The air passes through air filter and is fed into the evaporator with blower. Evaporator is multifunctional, which integrates evaporation, mixing, superheating and filtration. Gas of methanol, air, batching steam and off gas is proportionally mixed in the evaporator and then sentto the oxidation reactor. The advantage of the multifunctional evaporator is:
(1)The multifunctional evaporator may have replenishment of desalting water, reduce the consumption of batching stream and increase steam output.
(2)The multifunctional evaporator can apply the heat of circulating formaldehyde in the absorption column to heat methanol so as to save steam.
(3)Multifunctional evaporator can greatly shorten the distance between evaporator and reactor, making the equipment more compact and minimize the loss of heat.
Gas of methanol, air, batching steam and off gas is converted into formaldehyde with silver catalyst in the reactor. The reactor is equipped with a hot water heat exchanger. The purpose is:
(1)Increase the output of steam produced by the reactor, with each ton of formaldehyde, the byproduct of steam from the reactor is 550kg.
(2)Lower the temperature in reactor outlet and reduce the heat load of absorption column.
There are 2 absorption columns, one distillation section and two absorption sections in column one. It is to provide different gradient of concentration and temperature, not only to prevent formaldehyde polymerization but also to produce high concentration formaldehyde. Bubble cap
section is equipped at the top of the second absorption column, so as to enhance formaldehyde absorption and reduce methanol consumption.
Finally the off gas is sent to the off gas boiler from the top. There are three sections in the off gas boiler, including heat exchange section, hot water section and the air and off gas preheating section. The discharged gas from the boiler can preheat the boiler water, air and off gas for the
comprehensive utilization of the device. By-product steam from off gas boiler may be generated 400~450kg on the basis of each ton of formaldehyde.
Latest Dolton formaldehyde process flow chart

Process Comparison
Comparison of several silver formaldehyde processes

Comparison of metal oxide and silver catalyzed formaldehyde process
2,Methanol unit consumption: as the silver catalyzed process is prepared under the condition of excessive methanol, the methanol unit consumption of the silver catalyzed process is 15-20kg/t higher than that of metal oxide process.
3,Energy consumption: metal oxide process consumes 2.5 times more power than silver catalyzed process.
4,Steam output: steam output with metal oxide process is 100-150kg more per ton production of 37% formaldehyde than silver catalyzed process, and stream by-product of metal oxide process is 1.2mPa high quality saturated steam.
5,Catalyst service life: silver catalyst service life is normally about 3 months as it is reacted in above 630 ℃ high temperature, catalyst of metal oxide process with high activity normally may serve 12 to 18 months.
6,Product quality: both silver catalyzed process and metal oxide process can produce high concentration formaldehyde with low alcohol content. Formaldehyde concentration may be produced up to 55%.
- About Dolton & Helipont
- Formaldehyde production technology
- DMMn production technology
- Paraformaldehyde production technology
- Methylal production technology
- Hexamine production technology
- Acetaldehyde production technology
- Pentaerythritol production technology
- Trimethylolpropane production technology
- Neopentyl glycol production technology
- Trimeraldehyde production technology
- 1, 4-cyclohexane dimethanol CHDM production technology
- EPP production technology
- Production technology of concentrated formaldehyde made from dilute formaldehyde
